Research
Our group focuses on “electrolyte materials” that mediate redox reactions in electrochemical devices, with the goal of applying them to next-generation energy conversion and storage devices. In particular, we are investigating organic ion-conducting soft matter electrolytes such as concentrated electrolytes, ionic liquids, polymer electrolyte solutions, surfactant solutions, solid polymer electrolytes, and gel electrolytes, to exploit their unique functions of soft matter in storage batteries and other devices. The following is an example of a research topic currently under investigation.
Liquid electrolytes for next-generation batteries
The use of electric vehicles and stationary storage batteries is expected to continue to grow as they enable the stable use of renewable energy. To achieve this, it is essential to improve the performance of energy storage devices such as lithium-ion batteries. The characteristics required for next-generation energy storage devices include “energy density,” “rapid charge/discharge performance,” “safety,” “cost,” and “lifetime,” which are strongly influenced by the properties of electrolyte materials. Therefore, the development of superior electrolyte materials is essential for the practical application of next-generation energy storage devices.
Single Li-ion conducting liquid electrolytes
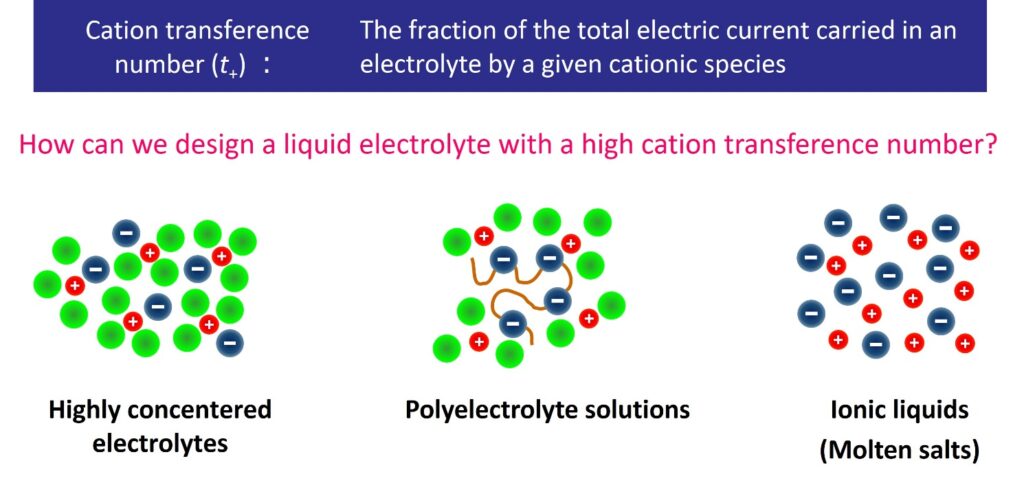
Energy density and rapid charge/discharge performance are greatly influenced by ionic conductivity and cation transference number, which are key parameters of electrolyte materials. For example, in a lithium-ion battery, the counter anion is not involved in the reaction, and only the Li ion is involved in the battery reaction. Therefore, electrolyte materials with both high ionic conductivity and high Li ion transference number are desired to achieve more efficient charge-discharge reactions.
Liquid electrolytes have been widely used in conventional batteries because the electrode-electrolyte interface can be easily formed. On the other hand, the liquid state allows both cations and anions to move freely. How can we create liquid electrolyte materials that can quickly and selectively carry only the ions involved in battery reactions? Our focus is on soft matter electrolytes such as concentrated electrolytes, polyelectrolyte solutions, and ionic liquids, with the goal of designing and developing liquid electrolytes that exhibit selective ionic conductivity through a precise understanding of the solvation and dynamics of ions in these solutions.
Reference:
K. Shigenobu et al, Chem. Rec., 2023, 23, e202200301.
Li-S battery electrolytes
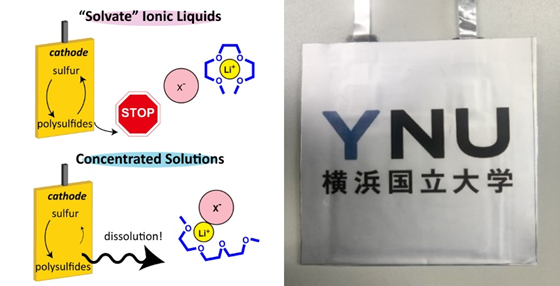
Lithium-sulfur (Li-S) batteries are attracting much attention as the next generation of energy storage due to their high energy density and expected low cost. On the other hand, there are many challenges to their practical application. One of them is that lithium polysulfides, which are formed as reaction intermediates in the sulfur electrode, dissolve in liquid electrolytes. When a conventional organic electrolyte is used, the charge-discharge cycle life of Li-S batteries is significantly reduced due to the dissolution of lithium polysulfides into the electrolyte.
Our group has developed a liquid electrolyte with extremely low solubility of lithium polysulfides using solvent-free ionic liquids and highly concentrated electrolytes with very high salt concentration. We are currently working on further improving the performance of the sparingly solvating electrolytes, as well as developing a prototype Li-S battery that actually exhibits high energy density, and investigating ways to extend the charge-discharge cycle life.
Reference:
S. Zhang et al., Adv. Energy Mater., 2015, 5, 1500117.
Soft materials based on ionic liquids
Currently, lithium secondary batteries use electrolytes consisting of Li salts in flammable organic solvents, but these can volatilize, leak, or catch fire, raising safety concerns as storage batteries continue to increase in size and energy density. Therefore, the development of thermally stable electrolyte materials such as ionic liquids, polymer electrolytes, and inorganic solid electrolytes is underway. Our group is conducting research on gel electrolyte materials, which are quasi-solid versions of ionic liquids and highly concentrated electrolytes. Gel electrolytes are not only solidified liquid electrolytes, but also have flexibility and rubbery elasticity unique to gel materials, and are expected to be used as electrolyte materials for new forms of energy storage devices such as flexible/stretchable batteries.
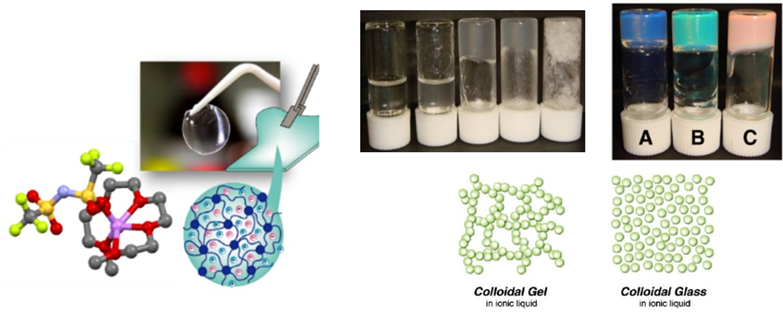
Gel electrolytes using polymers and nanofillers
Gel electrolytes behave as solid electrolytes while maintaining the same-level of ionic conductivity as liquid electrolytes, and have already been implemented in lithium-ion batteries. However, conventional gel electrolytes contain organic electrolytes, and the problem of flammability has not been completely solved. Our group has developed thermally stable gel electrolytes using ionic liquids and highly concentrated electrolytes, and investigated their ion transport, electrochemical, and mechanical properties in detail. We have found that ionic liquids and highly concentrated electrolytes can be quasi-solidified by combining them with polymers and inorganic nanoparticles, and are investigating material design and application to energy storage devices to achieve both high ion transport and mechanical properties.
References:
K. Ueno et al., Langmuir, 2011, 27, 9105.
Y. Kitazawa et al., Chem. Rec., 2018, 18, 391.
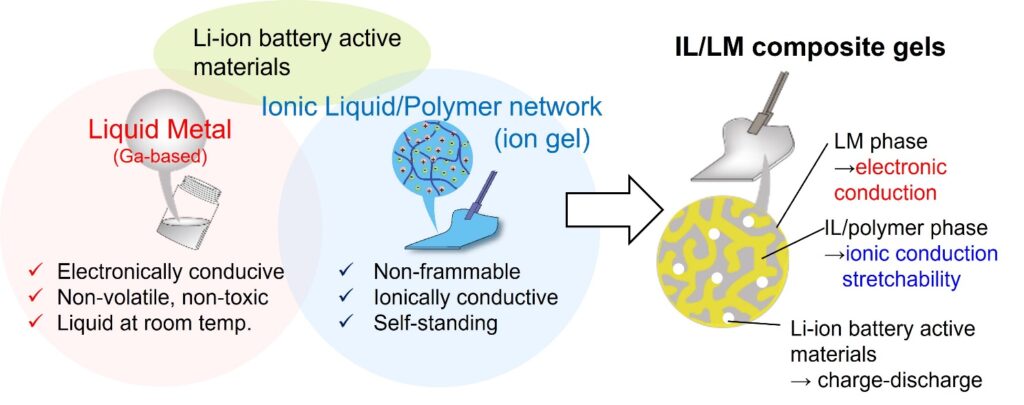
Mixed electronic-ionic soft conductors using liquid metals and ionic liquids
In recent years, with the development of Internet of Things (IoT) technology, research and development of flexible/stretchable devices and wearable devices designed to conform to the curved surfaces of structures and the human body has been active. Of course, a power source is needed to operate these devices, but the battery with sufficient flexibility and stretchability has not yet been achieved. Our group has developed a mixed electronic-ionic soft conductor (metal gel) by combining the ionic liquid-based gel electrolytes and Ga-based liquid metals. Furthermore, by adding lithium-ion battery active materials to this metal gel, we are investigating its application as a stretchable electrode for stretchable lithium-ion batteries.
Reference: J. Asada et al., Macromol. Chem. Phys., 2022, 223, 2100319.
Electrochemical CO2 capture, separation and conversion
To achieve carbon neutrality, there is growing demand for CCUS, which separates and captures CO2 from large emission sources such as thermal power plants and converts it into valuable resources or stores it underground. Electrochemical processes can be operated at room temperature and pressure and are attracting attention as an emerging technology with high energy efficiency.
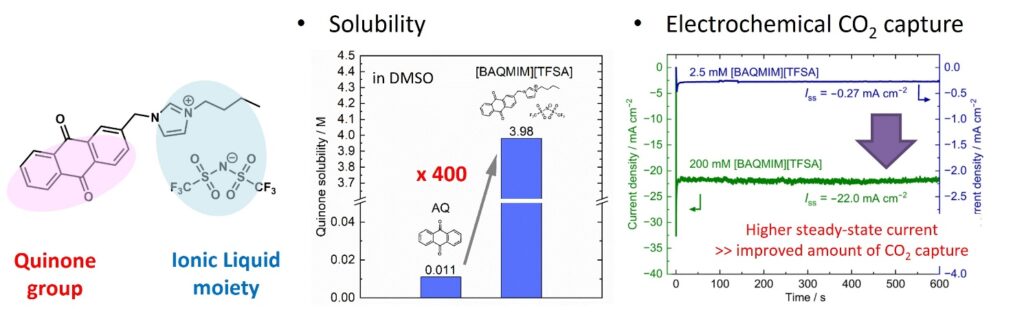
We particularly focus on quinones as electrochemical CO2 absorbers that are activated by redox reactions. The reduced forms of quinones are highly reactive to CO2. Our research group has designed ionic liquid-type functionalized anthraquinones by introducing polar groups to the quinone backbone. We have also demonstrated that these species have significantly increased solubility in solvents compared to conventional quinones. Currently, we are investigating further molecular design of quinone species and overall electrolyte design to achieve more efficient electrochemical CO2 capture, separation, and conversion reactions.
Reference:
H. Iida et al., J. Phys. Chem. C, 2023, 127, 10077.




